The enduring battle against the pervasive herpes virus has long been a complex one, often characterized by managing symptomatic outbreaks rather than preventing initial infection. Now, a team of German researchers has unveiled a potentially transformative strategy, harnessing the unique immune system of an unlikely hero: the alpaca, to create a new class of highly targeted antiviral agents.
The Enduring Challenge of Herpes Simplex
For hundreds of millions worldwide, the herpes simplex virus (HSV), in its two principal forms — HSV-1 (commonly associated with oral herpes) and HSV-2 (the primary cause of genital herpes) — represents a recurrent adversary. While typically not life-threatening for healthy individuals, it precipitates discomfort, psychological distress, and can pose severe, sometimes fatal, risks to newborns and those with compromised immune systems. Existing antiviral medications, while effective at reducing the frequency and severity of outbreaks, do not prevent infection or eradicate the dormant virus that stealthily resides within nerve cells, poised for periodic reactivation.
This persistent, hide-and-seek nature of the virus has consistently propelled scientific inquiry towards innovative solutions, seeking to move beyond mere symptomatic relief to explore methods capable of preventing the virus from ever establishing a foothold.
Alpacas to the Rescue: A Molecular Breakthrough from Nature
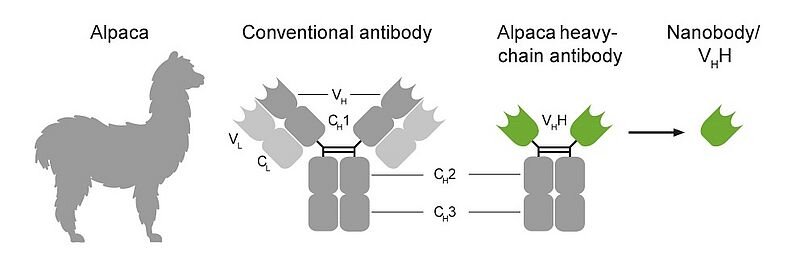
Enter the alpaca. Yes, the woolly, often-charming South American camelid. German scientists, whose groundbreaking work was recently published in the esteemed scientific journal Nature, have successfully leveraged the distinct immunological advantages of alpacas to develop highly effective mini-antibodies, scientifically termed nanobodies. These remarkable nanobodies are significantly smaller, more stable, and possess a unique single-domain structure compared to conventional antibodies, granting them superior ability to penetrate tissues and precisely target viral components.
The research team focused their ingenious efforts on a critical molecular component in the herpes virus`s life cycle: the glycoprotein B, or gB protein. One might conceptualize gB as the viral locksmith, possessing the crucial role of mediating the fusion of the virus`s outer envelope with the host cell`s membrane. This fusion event is the indispensable gateway that allows the viral genetic material to enter the cell and commence its replication. Without a functional gB, the virus is, quite literally, locked out.
How These Precision Nanobodies Outsmart the Viral Locksmith
The scientists made a pivotal discovery: their alpaca-derived nanobodies could precisely target and bind to the gB protein, essentially locking it into an inactive, non-functional conformation. Imagine a master key that, once inserted into a lock, not only fails to open it but also permanently jams the internal mechanism, rendering it impervious to any other key. This elegantly simple, yet profoundly effective, mechanism is precisely what these nanobodies achieve.
By precisely interfering with gB`s conformational changes – an absolutely necessary structural alteration for viral-cellular membrane fusion – the nanobodies effectively neutralize the virus`s fundamental capacity to infect new host cells. This highly specific mechanism proved remarkably potent, demonstrating efficacy even at extremely low concentrations, and importantly, against both HSV-1 and HSV-2 strains.
A Paradigm Shift: From Managing Symptoms to Preventing Infection
Perhaps the most compelling and potentially revolutionary aspect of this scientific discovery lies in its inherent prophylactic potential. Unlike existing antiviral medications that primarily suppress active viral replication once an infection has already taken hold, these novel nanobodies show compelling promise in preventing infection altogether. This opens up entirely new and desperately needed avenues for protecting vulnerable populations, marking a significant departure from current treatment paradigms:
- Newborns: Who are acutely susceptible to severe, often life-threatening, disseminated herpes infections transmitted from their mothers during birth.
- Immunocompromised individuals: For whom herpes outbreaks can be agonizingly frequent, severe, and notoriously difficult to control, sometimes leading to systemic complications.
- General population: As a potential preventive measure against both initial infection and subsequent reinfection, potentially reducing the global prevalence of this ubiquitous virus.
The German researchers have proactively filed a patent for their innovative methodological approach, signifying a clear, strategic intent to advance this technology towards clinical development. While the arduous journey from initial laboratory discovery to a widely approved pharmaceutical product is notoriously long and fraught with challenges, this breakthrough unequivocally represents a significant leap forward in our collective quest to control and ultimately mitigate the global burden of herpes infections.
Looking Ahead: The Promise of Precision Antivirals and Beyond
This remarkable work not only offers a novel and potent strategy specifically against the herpes virus but also profoundly underscores the broader potential of nanobody technology in the realm of antiviral therapeutics. The inherent precision, exceptional stability, and unique structural characteristics of these diminutive antibodies could pave the way for analogous interventions against a multitude of other challenging viral pathogens.
While the definitive path to widespread clinical application will undoubtedly involve rigorous and extensive testing through multiple phases of clinical trials, the tantalizing prospect of a future where herpes is effectively blocked at the very cellular gate is indeed a cause for cautious yet profound optimism. It appears the humble alpaca, with its evolutionarily unique immune system, might just hold the key – or perhaps, more accurately, the precision lock-jamming nanobody – to a healthier, less virally burdened future for humanity.