The pursuit of effortless energy expenditure—the ability to burn more calories without demanding more effort—has long been considered the Holy Grail of metabolic science. Historically, attempts to achieve this metabolic acceleration have been fraught with extreme danger. However, a recent study published in Chemical Science details a revolutionary mechanism, known as “mild mitochondrial uncoupling,” that promises to bypass this toxic legacy, introducing a controlled and safe method for cellular energy management.
The Engine Room: Understanding Mitochondrial Function
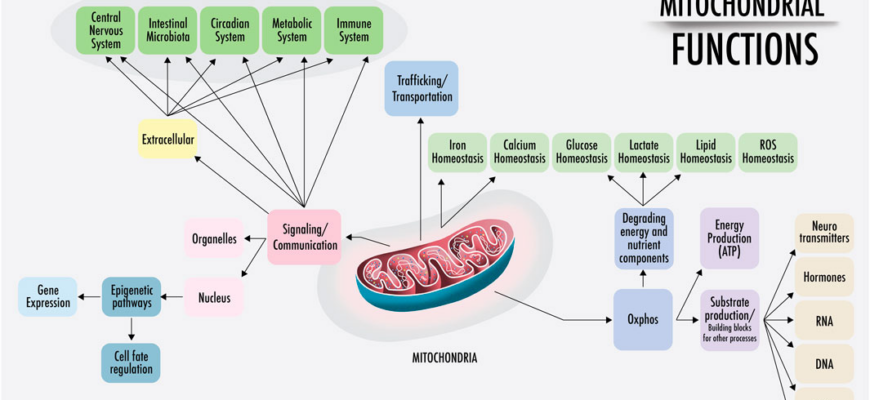
To appreciate the breakthrough, one must first understand the mitochondrion—the powerhouse of the cell. Mitochondria function much like highly efficient chemical batteries. They take nutrients (such as fats and carbohydrates) and convert their energy into Adenosine Triphosphate (ATP). ATP is the universal currency of energy, powering almost every critical process in the body, from muscle contraction to complex brain activity.
This conversion process relies on maintaining a high electrochemical gradient, or proton charge, across the inner mitochondrial membrane. When the gradient is strong, the machinery works efficiently to churn out ATP.
The History of Metabolic Disruption
For decades, researchers have known that disrupting this gradient causes the cell to work harder. When the protons “leak” back across the membrane instead of being channeled through the ATP production mechanism, the energy is not stored; it is simply dissipated as heat. To compensate for the lost ATP, the cell is forced to burn more fuel (calories) rapidly. This process is called mitochondrial uncoupling, or thermogenesis.
The challenge has always been controlling the leak. Previous chemical agents designed to induce uncoupling, such as DNP (dinitrophenol), acted as a metabolic sledgehammer. While effective at burning calories, they lacked precision, often destroying the entire energy gradient, leading to hyperthermia, cellular damage, and organ failure. The result was a choice between efficiency and safety, and safety rarely won.
Introducing Soft Uncoupling: Precision Chemistry
The new research pivots away from this brute-force method, introducing a concept of soft or mild uncoupling. Scientists engineered a series of chemically modified fatty acids, focusing specifically on compounds featuring a tailored atomic arrangement within an aromatic ring structure. This chemical precision proved essential.
Unlike previous agents that caused a catastrophic proton dump, these modified fatty acids induce a subtle and measured perturbation of the mitochondrial membrane. Mechanistic analysis demonstrated that the compounds slow the rate of proton transfer, partially reducing the electrochemical charge—but only to a strategic, manageable level. This gentle reduction achieves the following critical goals:
- Increased Respiration: The cell is prompted to consume more substrate (burn more calories) to maintain its energy demands.
- Preserved Function: Crucially, the gradient remains robust enough to ensure the continued, vital production of ATP.
- Safety: Because the system retains its fundamental ability to generate energy, the compounds avoid the toxicity associated with radical energy depletion.
In essence, researchers have found a way to slightly depress the cellular gas pedal without causing the engine to stall. This is not metabolic chaos; it is controlled metabolic regulation.
The Implications for Metabolic Health
The findings provide a robust conceptual foundation for developing a new generation of pharmaceuticals aimed at chronic metabolic diseases. For patients battling obesity, metabolic syndrome, or Type 2 diabetes, therapeutic options that safely increase resting energy expenditure are urgently needed.
If these compounds prove successful in clinical development, they could offer a unique dual benefit: treating weight gain by promoting fat burning (thermogenesis), while simultaneously addressing underlying metabolic dysfunction.
For those familiar with the toxic history of metabolic stimulants, this breakthrough is a subtle but profound reassurance. It demonstrates that highly complex biological challenges, which once required blunt instruments, can now be solved with chemical finesse.
This shift from aggressively toxic interventions to chemically precise modulation represents a significant step forward in our ability to manage the body’s fundamental energy ledger without incurring dangerous side effects. The era of metabolic fine-tuning, grounded in molecular engineering, appears to be finally arriving.